Cardioskin

- Publisher: Les Laboratoires Servier
- Genre: Medical
- Released: 18 Nov, 2019
- Size: 39.9 MB
- Price: FREE!
 Click here to request a review of this app
Click here to request a review of this app
- App Store Info
Description
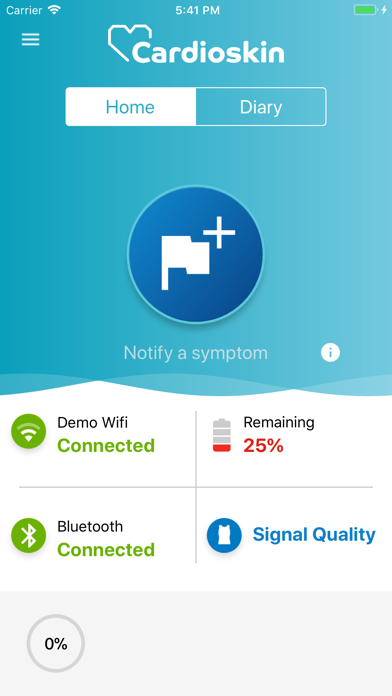
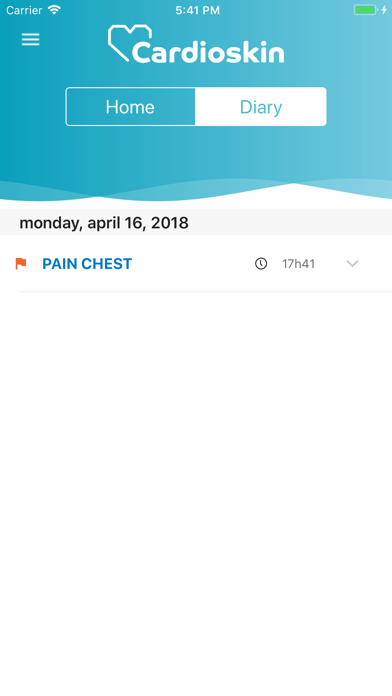
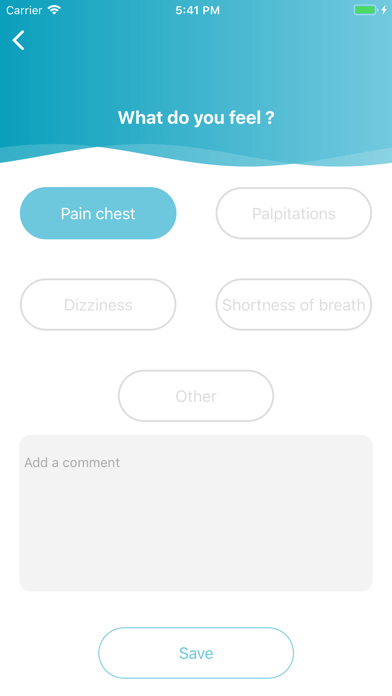
Cardioskin Application is medical device that enables acquisition, recording, storage, transmission, and display of 15-leads electrocardiogram (ECG) in order to analyze potential cardiac pathological abnormalities.Cardioskin Application is available:
- In Europe, as a class IIa medical device. It is a regulated health product that bears, under the European regulation of medical devices (93/42 / EEC), the CE 2797 mark.
- In the United States, as a Class I medical device. It is a regulated health product that is, under the United States Medical Device Regulations (21 CFR part 800), exempt from 510 (k) clearance.
Cardioskin Application cannot be used alone, it is part of the Cardioskin system and federal law restricts this device to sale by or on the order of a physician.
The application is intended for healthcare professionals and their patients over 15 years in Europe and over 18 years in USA.
Users are reminded to:
- Carefully read the instructions supplied with the product before use;
- Consult a healthcare professional if in doubt and before making medical decisions.
More information available at: https://www.bioserenity.com/en/quality-and-regulatory/.
This application is developed by BioSerenity, ICM-iPEPS, 47 boulevard de l’hôpital, 75013 Paris - FRANCE.
The current version available is V.2.6.1 [ref: 5100-00008] approved in November 2020
LAB-00301 rev A
What's New in Version 2.6.1
We update the app regularly to improve your experience. This update includes:Daily reminder notifications for patients
User manuel and regulatory information access from “About” page
Bug fixes
Performance optimization